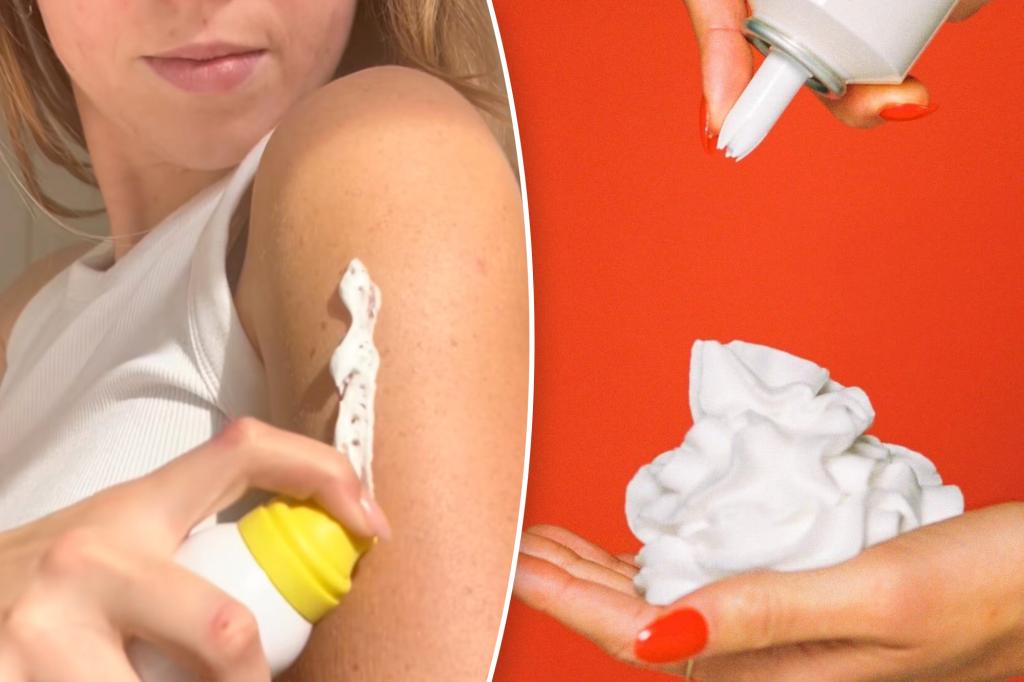
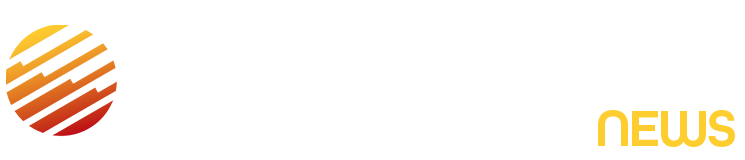Some of the summer’s hottest beauty products just got burned.
“Beware of sunscreen products in mousse form because they might not be effective,” the Food and Drug Administration cautioned in a post on X this week.
The alert follows a wave of warning letters the agency sent to Supergoop!, Vacation Inc. and three other popular brands, accusing them of peddling whipped, mousse and foam sunscreens that flout federal laws.
Before you send your beloved sunscreens packing, let’s break down the FDA’s warnings.
The letters — also sent to Kalani Sunwear, K & Care Organics and Fallien Cosmeceuticals (makers of TiZo sunscreen) — claim the frothy formulas are “misbranded.”
That’s because sunscreens are classified as over-the-counter drugs in the US — and that comes with strict regulations around how they’re formulated and marketed.
Per FDA rules, sunscreens can only be sold as oils, lotions, creams, gels, butters, pastes, ointments, sticks, sprays or powders — not foams, mousses, or whips.
To use those formats legally, companies must submit a new drug application with sufficient data proving their safety and effectiveness.
“There are no FDA-approved applications in effect for your drug products,” the agency wrote.
The FDA also took aim at Vacation Inc.’s “Classic Whip Sunscreen,” sold in containers that look like whipped cream cans and advertised as “dessert for your skin.”
“Packaging drug products in containers that resemble food containers commonly used by adults and children can mislead consumers into mistaking the products for food, which is of particular concern as this increases the risk of accidental ingestion,” the agency warned.
But the FDA’s heat doesn’t necessarily mean these fluffy formulas fall short at blocking burns or reducing skin cancer risk.
“The letter does not state that mousse-format sunscreens are less effective or unsafe, nor does it question the quality or performance of our formulation,” Kalani Sunwear told Cosmetics Business, adding that it’s “Sun Mousse SPF 50 is developed and manufactured in Sweden to the highest European standards.”
A Supergoop! spokesperson told The Post that the warning “is focused on product labeling and has nothing to do with its safety, effectiveness or formula,” and added that the company is “working closely with the FDA to resolve this matter.”
Still, some outside experts aren’t so sure about the whipped format.
“SPF is determined by applying 2 mg/cm^2, which is measured by weight, not volume,” Ava Perkins, a cosmetic chemist and US sunscreen expert, told The Cut.
“Because these mousse sunscreens have so much air incorporated into the product, it can be challenging to know if you’re putting on enough, even if it seems like a lot is being dispensed,” she added.
The FDA’s letters, dated August 6, gave each company 15 working days to explain how they plan to fix the issues or prove they’re not in violation.
Kalani Sunwear has already started taking action, temporarily pulling its mousse-format sunscreen from its US website “to ensure full compliance with the regulations,” according to CBS News.
In a statement to The Post, a spokesperson for Vacation said that the company takes “regulatory compliance seriously.”
“We have full confidence in the safety, efficacy, and integrity of our product,” they added. “We are committed to working collaboratively with the FDA to satisfactorily resolve this matter.”
The Post has reached out to Kalani Sunwear, K & Care Organics and Fallien Cosmeceuticals for comment.
Read the full article here